Small-scale synthesis in laboratory settings offers efficient, cost-effective, and sustainable methods for preparing reagents, as highlighted in Leonid Lerner’s work. This approach minimizes waste and enhances customization, making it a valuable tool for chemists and researchers.
Importance of Small-Scale Synthesis in Laboratory Settings
Small-scale synthesis is crucial in laboratory settings as it enables the efficient preparation of high-purity reagents with minimal resource consumption. This approach reduces reliance on commercial suppliers, often eliminating long wait times and high costs. By synthesizing reagents in-house, researchers gain control over quality and customization, ensuring reagents meet specific experimental needs. Additionally, small-scale methods minimize waste, aligning with sustainable laboratory practices. They are particularly valuable for producing rare or hard-to-obtain chemicals, fostering innovation and flexibility in scientific workflows. This technique also enhances safety by reducing the handling of hazardous materials in large quantities. Overall, small-scale synthesis streamlines laboratory operations, making it a cornerstone of modern chemical research and education.
Overview of Leonid Lerner’s Book
Leonid Lerner’s book, Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling, provides a comprehensive guide for chemists, researchers, and educators. It focuses on efficient methods for synthesizing reagents in laboratory settings, emphasizing small-scale production to minimize costs and environmental impact. The book is enriched with practical techniques and mathematical models to predict and optimize reaction outcomes. Lerner addresses the challenges of obtaining rare or expensive reagents, offering alternative synthesis routes. This resource is particularly valuable for academic and research environments, where customization and purity of reagents are critical; By integrating reaction modeling, the book bridges theory and practice, making it an essential tool for advancing chemical synthesis and education;

Advantages of In-Lab Preparation of Reagents
In-lab preparation of reagents offers cost savings, reduced environmental impact, and customization of properties, making it a practical and sustainable alternative to commercial sources.
Cost-Effectiveness and Resource Efficiency
In-lab preparation of reagents significantly reduces costs by eliminating reliance on commercial suppliers. Small-scale synthesis allows laboratories to produce only what is needed, minimizing waste and optimizing resource use. This approach is particularly beneficial for reagents that are expensive or difficult to obtain commercially. By utilizing readily available chemicals and simple equipment, labs can achieve cost savings while maintaining high purity standards. Additionally, small-scale methods often require less storage space and reduce the environmental impact associated with transportation and packaging. This resource-efficient approach makes it accessible for laboratories with limited budgets to sustain their operations effectively.
Customization of Reagent Properties
One of the key advantages of in-lab reagent synthesis is the ability to tailor reagents to specific needs. By adjusting reaction conditions, such as temperature, concentration, and solvent, scientists can customize properties like purity, stability, and reactivity. This level of control is often unattainable with commercial products, which may not meet the exact requirements of a particular experiment. Customization also allows researchers to avoid impurities present in commercial reagents that could interfere with reactions. Small-scale synthesis methods, as detailed in Leonid Lerner’s work, provide precise control over the synthesis process, enabling the creation of reagents with specific characteristics. This flexibility is invaluable for advancing research and ensuring experimental accuracy.
Environmental Benefits of Reduced Waste
Small-scale synthesis significantly reduces waste by minimizing excess chemical production. Unlike commercial reagents, which often come in bulk quantities, in-lab synthesis produces only what is needed, lowering the volume of unused materials. This reduction in chemical waste contributes to a more sustainable laboratory practice. Additionally, smaller reaction volumes decrease the amount of packaging and transportation-related waste. By optimizing reagent preparation, laboratories can minimize their environmental footprint while maintaining high standards of research quality. Leonid Lerner’s methods emphasize efficient resource use, aligning with green chemistry principles and promoting eco-friendly laboratory practices.
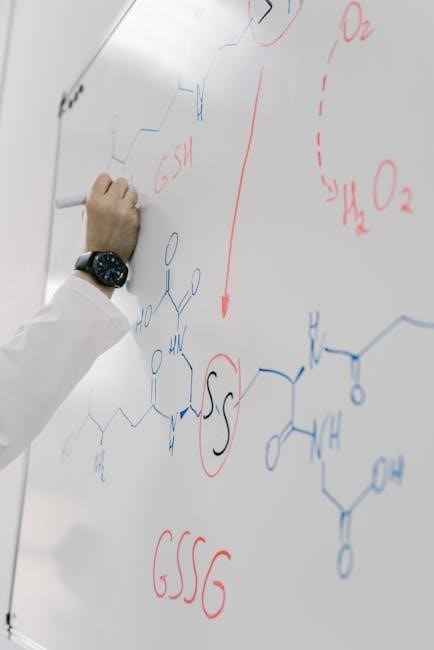
Key Concepts in Small-Scale Synthesis
Small-scale synthesis involves precise reaction modeling, efficient use of resources, and essential laboratory equipment. It focuses on optimizing chemical reactions to produce high-purity reagents with minimal waste.
Reaction Modeling Basics
Reaction modeling is a cornerstone of small-scale synthesis, enabling chemists to predict and optimize reaction outcomes. By using mathematical models, researchers can simulate how reagents interact under specific conditions, reducing trial-and-error experiments. These models incorporate variables like temperature, concentration, and reaction time to forecast yields and identify potential side reactions. Advanced simulation tools, as discussed in Leonid Lerner’s work, allow for precise control over synthesis parameters, ensuring high-purity products. This approach not only streamlines the synthesis process but also minimizes waste and resource consumption, making it a sustainable and efficient method for laboratory reagent preparation. Effective reaction modeling is essential for achieving consistent and reliable results in small-scale synthesis.
Essential Laboratory Equipment and Setup
The proper setup of laboratory equipment is crucial for successful small-scale synthesis. Key tools include glassware such as round-bottom flasks, beakers, and test tubes, which are essential for mixing and heating reagents. Heating and cooling systems, like hot plates or ice baths, are necessary for controlling reaction temperatures. Stirring devices, such as magnetic stir bars and stir plates, ensure uniform mixing. Precision instruments like pipettes and burettes are used for accurate measurements. Safety equipment, including gloves, goggles, and fume hoods, is vital for handling hazardous chemicals. A well-organized workspace with access to these tools enables chemists to efficiently synthesize reagents while maintaining safety and precision, as outlined in Leonid Lerner’s comprehensive guide.

Categories of Laboratory Reagents
Laboratory reagents are categorized into inorganic, organic, and specialized types, each serving distinct roles in chemical synthesis, as detailed in Leonid Lerner’s comprehensive guide.
Inorganic Reagents
Inorganic reagents play a crucial role in small-scale synthesis, offering foundational chemical building blocks for various laboratory processes. These reagents, such as acids, bases, and salts, are often used in acid-base reactions, precipitation, and oxidation-reduction processes. Their simplicity and stability make them ideal for cost-effective synthesis. Many inorganic reagents can be prepared in-house, reducing reliance on commercial suppliers and enabling customization. Leonid Lerner’s work highlights methods for synthesizing these reagents efficiently, ensuring high purity and consistency. These compounds are essential for experimental setups, providing reliable results in both teaching and research environments. Their versatility and widespread application make them indispensable in modern chemical laboratories.
Organic Reagents
Organic reagents are essential in small-scale synthesis due to their versatility and application in various chemical reactions. These compounds, often complex molecules, are frequently synthesized in laboratory settings to meet specific experimental needs. Small-scale preparation allows for precise control over reagent properties, such as purity and functionality, which is critical for achieving desired reaction outcomes. Leonid Lerner’s work emphasizes efficient methods for synthesizing organic reagents, leveraging common laboratory equipment and minimal starting materials. This approach not only reduces costs but also enables customization, making it particularly valuable for research and educational purposes. The ability to produce organic reagents in-house ensures timely availability and adapts to the dynamic demands of chemical experimentation, fostering innovation and practical learning in laboratory environments.
Specialized Reagents for Specific Reactions
Specialized reagents are tailored for unique chemical reactions, offering precise functionality and high efficiency. These reagents are often required in small quantities and may involve complex synthesis processes. Leonid Lerner’s work highlights methods for preparing such reagents, emphasizing their role in advancing specific reactions. Small-scale synthesis allows for the customization of these reagents, ensuring they meet the exact demands of experimental conditions. This approach is particularly valuable when commercial alternatives are unavailable or impractical. By focusing on targeted reagent design, researchers can achieve superior reaction outcomes, enabling breakthroughs in organic and inorganic chemistry. The ability to model and optimize these reagents further enhances their utility in laboratory settings, making them indispensable for specialized applications.

Role of Reaction Modeling in Synthesis
Reaction modeling plays a crucial role in synthesis by enabling precise prediction of outcomes, optimizing reaction conditions, and integrating with laboratory techniques for efficient reagent preparation.
Mathematical Modeling Techniques
Mathematical modeling techniques are essential in small-scale synthesis for predicting reaction outcomes and optimizing conditions. These methods involve creating detailed mathematical representations of chemical reactions, incorporating kinetic parameters and thermodynamic data. Computational tools simulate reaction pathways, enabling researchers to explore variables like temperature, concentration, and catalysts. This approach reduces trial-and-error experiments, saving time and resources. Advanced models, such as those described by Leonid Lerner, integrate experimental data with theoretical frameworks to enhance accuracy. By leveraging these techniques, chemists can design efficient synthesis protocols and scale processes effectively. Mathematical modeling also facilitates the identification of potential challenges, ensuring safer and more reliable laboratory practices. Its application is particularly valuable in educational settings, teaching students fundamental principles of chemical synthesis and reaction dynamics.
Use of Simulation Tools for Prediction
The use of simulation tools is a cornerstone of modern small-scale synthesis, enabling researchers to predict reaction outcomes with high accuracy. These tools leverage advanced algorithms to simulate reaction dynamics, helping chemists anticipate challenges and optimize conditions. Simulation software can model variables such as temperature, pressure, and solvent effects, providing insights into reaction pathways and potential byproducts. By integrating experimental data, these tools enhance the reliability of predictions, reducing the need for costly trial-and-error experiments. Leonid Lerner’s work emphasizes the importance of simulation in streamlining synthesis processes, particularly for complex reactions. This approach not only improves efficiency but also supports safer laboratory practices by identifying potential hazards before experimentation begins.
The Synthesis Process
The synthesis process involves planning routes, executing protocols, and optimizing conditions to achieve desired reagents efficiently. Small-scale methods enhance precision and reduce resource consumption, ensuring successful outcomes.
Planning and Designing Synthesis Routes
Planning and designing synthesis routes involves evaluating starting materials, reaction pathways, and conditions to achieve optimal results. Leonid Lerner’s methods emphasize efficient reaction modeling, enabling chemists to predict outcomes and minimize trial-and-error. By customizing synthesis routes, researchers can tailor reagent properties to specific needs, ensuring high purity and yield. This step also involves assessing resource availability and safety protocols, making it a critical phase in small-scale synthesis. Advanced tools and simulations aid in streamlining the process, reducing costs and environmental impact. Effective route design ensures experiments are reproducible and scalable, aligning with laboratory goals and constraints.
Execution of Synthesis Protocols
Execution of synthesis protocols involves carrying out planned reactions using optimized conditions and equipment. Small-scale synthesis often employs common laboratory tools, ensuring accessibility and efficiency. Researchers follow detailed step-by-step procedures to maintain consistency and reproducibility. Real-time monitoring of reactions allows for immediate adjustments, enhancing yield and purity. Safety protocols are critical, as hazardous chemicals may be involved. Proper handling and waste disposal methods are emphasized to minimize environmental impact. Documentation of each step ensures transparency and facilitates future replication. By adhering to these practices, chemists can reliably produce high-quality reagents, aligning with the practical approaches outlined in Leonid Lerner’s work.
Optimization of Reaction Conditions
Optimizing reaction conditions is crucial for achieving efficient and reproducible synthesis outcomes. Small-scale synthesis allows for rapid testing of various parameters, such as temperature, solvent choice, and reaction time, to identify ideal settings. Mathematical modeling, as discussed in Leonid Lerner’s work, provides insights into how these variables interact, enabling predictive adjustments. Iterative testing and real-time monitoring of reactions help refine conditions to maximize yield and purity. This process not only enhances the quality of reagents but also reduces waste and conserves resources. By systematically evaluating and refining reaction conditions, chemists can develop robust protocols tailored to specific needs, ensuring reliability and scalability in laboratory settings.
Safety Considerations
Handling hazardous chemicals requires strict adherence to safety protocols, including proper PPE and ventilation. Proper waste disposal and emergency preparedness are essential to minimize risks in laboratory settings.
Handling Hazardous Chemicals
Handling hazardous chemicals in small-scale synthesis requires meticulous attention to safety protocols to minimize risks. Proper personal protective equipment (PPE), including gloves, goggles, and lab coats, is essential. Ensuring adequate ventilation in the laboratory setting is critical to prevent inhalation of toxic fumes. Chemicals should be stored according to their hazard classifications, and emergency response plans must be in place. The use of fume hoods and spill containment measures further enhances safety. Leonid Lerner’s work emphasizes the importance of understanding chemical reactivity and potential hazards before initiating synthesis. Regular training and adherence to safety guidelines are vital to protect both personnel and the environment. Proper labeling and disposal of hazardous waste are also integral to maintaining a safe laboratory environment.
Proper Waste Disposal Methods
Proper waste disposal is crucial in small-scale synthesis to minimize environmental impact and ensure laboratory safety. Waste should be segregated into categories such as hazardous, non-hazardous, organic, and inorganic materials. Each type must be stored in appropriately labeled containers to prevent contamination. Disposal should follow local and institutional regulations, with hazardous waste requiring special handling, such as incineration or chemical neutralization. Regular training on waste management practices is essential for laboratory personnel. Additionally, maintaining detailed records of waste generation and disposal ensures compliance with environmental guidelines. Proper waste disposal not only protects the environment but also aligns with sustainable laboratory practices emphasized in Leonid Lerner’s approaches to small-scale synthesis.

Applications in Education
Small-scale synthesis is a valuable teaching tool, enabling chemistry students to gain hands-on experience with reagent preparation and reaction modeling, fostering practical skills in laboratory settings.
As a Teaching Tool for Chemistry Students
Small-scale synthesis serves as an invaluable educational resource, enabling chemistry students to explore reagent preparation and reaction modeling hands-on. This approach bridges theoretical knowledge with practical application, fostering a deeper understanding of chemical principles. By engaging in synthesis processes, students develop critical problem-solving skills and attention to detail, essential for laboratory work. The simplicity and efficiency of small-scale methods make them ideal for academic settings, allowing educators to demonstrate complex reactions safely and effectively. Leonid Lerner’s work provides a comprehensive guide, offering step-by-step protocols and mathematical models that simplify learning. This teaching tool not only enhances student engagement but also prepares future chemists for real-world laboratory challenges, making it a cornerstone of modern chemistry education.
Hands-On Projects for Undergraduate Labs
Small-scale synthesis is particularly well-suited for undergraduate laboratory projects, offering practical experience in reagent preparation and reaction modeling. These projects are cost-effective and utilize common laboratory equipment, making them accessible for educational settings. Students can synthesize reagents quickly, often in a single lab session, while gaining insights into chemical reactions and safety protocols. Such hands-on activities are ideal for teaching fundamental principles of chemistry, such as stoichiometry, purification, and characterization. By engaging in these projects, undergraduates develop essential laboratory skills, including accurate measurements and data analysis. These exercises also foster problem-solving abilities and teamwork, aligning with educational goals. Small-scale synthesis projects thus serve as a bridge between theory and practice, preparing students for advanced research and real-world applications in chemistry.
Applications in Research
Small-scale synthesis facilitates advancements in organic chemistry by enabling efficient reagent preparation and reaction modeling, as detailed in Leonid Lerner’s work, driving innovation in research settings.
Advancements in Organic Chemistry
Small-scale synthesis has revolutionized organic chemistry by enabling the efficient preparation of high-purity reagents and complex molecules. Leonid Lerner’s methods, as detailed in his work, provide chemists with precise control over reaction conditions, allowing for the creation of specialized reagents that were once difficult to obtain. This approach not only accelerates discovery but also reduces the need for large-scale production, making it environmentally friendly. By integrating mathematical modeling and experimental techniques, researchers can optimize synthesis routes and predict outcomes more accurately. These advancements have opened new avenues for exploring novel chemical reactions and compounds, fostering innovation in organic synthesis and its applications across various scientific fields.
Case Studies and Successful Implementations
Leonid Lerner’s methods have been successfully applied in various laboratory settings, demonstrating the practicality of small-scale synthesis. For instance, the preparation of salicylic acid, a common organic compound, has been streamlined using these techniques. Researchers have achieved high-purity yields with minimal equipment, showcasing the efficiency of in-lab reagent synthesis. Additionally, case studies highlight the use of mathematical modeling to predict reaction outcomes, enabling precise control over synthesis parameters. These implementations not only reduce costs but also minimize waste, aligning with sustainable laboratory practices. Successful examples from Lerner’s work illustrate how small-scale synthesis can be effectively integrated into both educational and research environments, providing valuable insights for chemists and educators alike.

Future Trends and Challenges
Technological advancements, like AI-driven synthesis tools, promise to enhance small-scale synthesis efficiency. However, scaling these methods to industrial levels while maintaining cost-effectiveness remains a significant challenge.
Impact of Technological Advancements
Technological advancements are revolutionizing small-scale synthesis, offering unprecedented efficiency and precision. Tools like AI-driven synthesis planners and automated laboratory equipment enable rapid optimization of reaction conditions. Mathematical modeling, as discussed in Leonid Lerner’s work, is being enhanced by machine learning algorithms, improving predictive capabilities. These innovations reduce trial-and-error processes, accelerate discovery, and minimize resource waste. Additionally, real-time monitoring systems allow for better control over reaction dynamics, ensuring higher purity and yield of reagents. Such technologies not only streamline laboratory workflows but also open new avenues for scaling up synthesis methods while maintaining cost-effectiveness. The integration of digital tools with traditional synthesis techniques promises to transform laboratory practices, making them more sustainable and efficient for future researchers.
Challenges in Scaling Up Synthesis
Scaling up small-scale synthesis to industrial levels presents significant challenges, including maintaining reaction consistency and controlling variables like temperature and concentration. As highlighted in Leonid Lerner’s work, laboratory methods often rely on precise conditions that are difficult to replicate on a larger scale. Equipment limitations, such as heat transfer inefficiencies in industrial reactors, can lead to reduced yields or impurities. Additionally, the cost of scaling up can be prohibitive, requiring specialized equipment and expertise. Ensuring the stability and purity of reagents during larger production runs further complicates the process. Addressing these challenges demands innovative solutions, including advanced modeling techniques and robust process optimization strategies to bridge the gap between laboratory and industrial synthesis.
Small-scale synthesis is a vital approach in modern chemistry, offering cost-effective and environmentally friendly solutions. Leonid Lerner’s insights highlight its importance in advancing laboratory practices and sustainability.
Small-scale synthesis in laboratory settings has emerged as a cost-effective and environmentally friendly approach for preparing reagents. It minimizes waste, reduces reliance on commercial suppliers, and allows for customized reagent properties. Leonid Lerner’s work emphasizes the importance of reaction modeling in optimizing synthesis protocols, ensuring high-purity products with minimal resource consumption. The integration of mathematical modeling and simulation tools enhances predictability and efficiency in chemical synthesis. This method is particularly valuable for educational purposes, providing hands-on experience for students and researchers. By focusing on sustainable practices and precise control over reaction conditions, small-scale synthesis stands as a cornerstone of modern laboratory chemistry, offering practical solutions for both research and education.
The advantages of in-lab reagent preparation, as outlined in Lerner’s book, include reduced costs, improved resource efficiency, and the ability to tailor reagents to specific experimental needs. Environmental benefits, such as reduced waste and lower chemical consumption, further underscore its importance. The use of essential laboratory equipment and modeling techniques ensures that small-scale synthesis remains accessible and scalable. Overall, this approach not only advances chemical research but also fosters a deeper understanding of reaction dynamics, making it a vital tool for chemists and educators alike.
Final Thoughts on the Importance of Small-Scale Synthesis
Small-scale synthesis represents a transformative approach in laboratory chemistry, offering practical, cost-effective, and environmentally sustainable methods for reagent preparation. By minimizing waste and reducing reliance on commercial suppliers, this technique aligns with modern demands for efficiency and sustainability. Leonid Lerner’s work underscores its versatility, enabling researchers to customize reagents for specific needs while maintaining high purity. The integration of reaction modeling further enhances its value, providing predictive insights that streamline synthesis processes. As a teaching tool, it equips students with hands-on experience, bridging theory and practice. Ultimately, small-scale synthesis is not just a method but a cornerstone of innovative and responsible chemical research, driving advancements while fostering a deeper understanding of reaction dynamics.
